The urban heat island (UHI) effect.
The conventional wisdom is that climate change is driven by rising carbon dioxide (CO2) levels in the atmosphere, and only by CO2; so the greater the (CO2) levels the greater the temperature increase (see Fig. 87.3 in Post 87). You may have noticed one direct consequence of this orthodoxy in the way the media these days reports on environmental disasters or extreme weather (floods, droughts, storms, hurricanes, heatwaves, forest fires etc.): they always refer to climate change.
Implicit to this climate change reference is the assumption that all climate change is due to CO2 even though CO2 is rarely explicitly mentioned and the causation is rarely demonstrated. Consequently, the solution to all extreme weather events appears to be simple and obvious: cut CO2 levels in the atmosphere (i.e. Net Zero) and everything will be fine. Except it won't. This is because much of what is happening to local climates has little or nothing to do with CO2, but it does have a lot to do with other human activities, not least urbanization and industrialization. Central to both of these is the urban heat island (UHI) effect.
The problem when discussing the impact of the UHI effect on climate, and in particular the temperature record, is that it is controversial. This is partly because much of climate science appears to be driven by an anti-fossil fuel dogma that therefore sees any talk of UHIs as at best a distraction from the supposed only true problem, CO2, and at worst a campaign of disinformation designed to undermine all of climate science and its campaign against CO2. But it is also partly because UHIs come in many flavours.
There are those UHIs that just trap more heat by reducing airflows and those that store more solar heat than rural areas by virtue of increased heat capacities. Both of these do not add to the total amount of energy absorbed at the Earth's surface though, so there is no net global temperature increase associated with them. But then there are those UHI processes that do absorb extra heat, either via changes to the albedo of the Earth's surface, or by the emission of large amounts of additional heat through anthropogenic energy use and generation. Both of these certainly do add to global warming but are still largely ignored by climate science. In the following sections I will discuss the relative impact of each of these four types of UHI effect in turn and show that one type in particular can be very significant.
i) Heat trapping
The aspect of the UHI effect that is referred to the most is heat trapping. This is where tall buildings in a city reduce the flow of hot air away from the centre causing the city to retain its heat longer. Perhaps the most obvious example of this is Manhattan in New York City with its dense cluster of tall skyscrapers.
The result of this UHI effect is that the local area of the city stays hotter for longer compared to if the buildings were not there. This is because there is less diffusion of heat to outlying areas, so those areas are less likely to be warmed by the city and the city is less likely to be cooled by heat transfer to the rural areas that surround it.
However, this does not lead to more global warming because while the city will be hotter for longer than otherwise expected, the surrounding area will be cooler for longer as well because less heat from the urban areas reaches the rural areas. The key point here is that no extra heat is created at the surface of
the Earth, it is just prevented from diffusing to colder regions. So the net effect on local mean temperatures is zero. As an example consider the Grand Canyon. It will trap heat in the same way that tall buildings do, but does that mean that it is warming faster than the rest of Arizona? No, and nor does it make Arizona as a whole get any warmer.
This is one reason why climate scientists discount the UHI effect, and in this case they are right, provided that the weather stations used to monitor temperature changes are evenly distributed and their temperature readings are not adjusted. Those, unfortunately, are big IFs, because any bias in station numbers between urban and rural regions compared to their relative areas will affect the the relative contribution of each to the mean global temperature, and we do know that station densities are generally higher in urban areas. So potentially there are more warm urban stations contributing to the global average than there should be and fewer cold rural ones. In an ideal world, though, this should not occur, and so neither would any contribution to global temperatures.
Net effect on global warming: zero.
ii) Increased heat capacity
Probably the second most cited variation of the UHI effect is heat retention where cities heat up and store energy from the Sun during the day and then gradually release it overnight. The net effect of this is that the maximum temperature in the city during the day should be less than expected because of the time it takes the buildings to heat up. This is because the Sun is not just heating up the top layer of the Earth's surface, as would be the case in rural areas; it is also having to heat up large concrete structures with much higher heat capacities. The higher the local heat capacity of these structures, the longer it takes to warm them and the slower, and therefore lower, their temperature rise will be. This in turn means that less infra-red radiation is then radiated back into outer space during the day because the region is cooler than it would be without the buildings, and so there is less heating of the lower atmosphere and less downwelling radiation.
At night, however, the heating from the Sun stops. The rural areas cool quickly but the urban areas don't because the urban areas have the much higher heat capacity: there is more heat stored that needs to be lost before a new thermal equilibrium without the Sun can be established. So the buildings are now warmer than their rural surroundings but are slowly cooling, acting like large radiators or storage heaters. This means that the city stays warmer for longer, and temperatures within the city are higher at night than they would otherwise be.
The net effect of this is that temperatures during the day will be lower, but those at night-time will be higher. Overall, though, the effect on the average temperature will be zero as the two changes in temperature cancel due to the fact that the changes in heat absorption will also cancel.
Net effect on global warming: zero.
iii) Increased heat absorption
One consequence of urban development is that it changes the reflectivity of the Earth's surface for incident visible, ultraviolet and near infra-red radiation. This reflectivity is known as the albedo and it is loosely related to the colour of a surface: darker colours tend to absorb more radiation while lighter ones generally reflect more. If the albedo increases, then more radiation is reflected back into space without heating the planet, so ice and snow help to cool the planet (their albedo is over 80%) while dark soil and oceans tend to warm the planet (see Table 14.1 in Post 14 for a list of typical albedos). It therefore follows that if the colour of a surface changes, then so will its albedo, and this can then change the amount of radiation absorbed at the surface. If this absorbed radiation increases, then the Earth will get warmer and the UHI effect is one way this can happen.
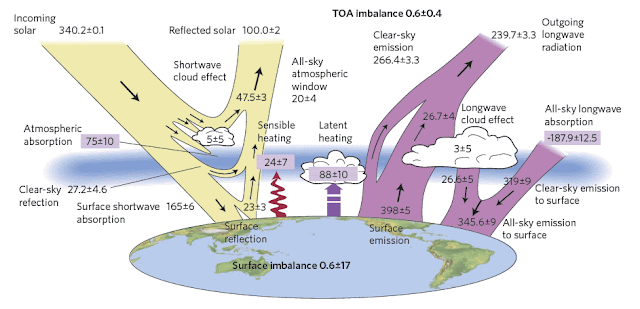
In Post 14 I explained that of the average incoming solar radiation of 341 W/m2 that the Earth receives, only 161 W/m2 is absorbed at its surface, and that greenhouse gases then amplify this with 333 W/m2 of additional downwelling radiation. This total absorbed heat of 494 W/m2 then dictates the mean surface temperature via the Stefan-Boltzmann law (see Post 12). It therefore follows that if any change occurs at the Earth's surface that increases the 161 W/m2 of absorbed radiation, then this will change the downwelling radiation by the same percentage and therefore change the mean surface temperature as well.
The process of urbanization inevitably involves changing the colour and texture of the Earth's surface. It generally means that areas of vegetation are replaced with tarmac and concrete. Buildings with dark roofs absorb more solar radiation than trees and grassland. However the situation is not straightforward because concrete can be very reflective and arable land tends to be very dark. Overall though, there is generally a small decrease in albedo with urbanization, and therefore a small increase in the amount of solar radiation that is absorbed. This will raise the surface temperature of the Earth slightly as well, but because it is small it is not likely to be significant.
One human innovation that can have a big impact on temperature is solar power. Because solar panels are designed to absorb 99% of solar radiation, they will add additional heating to any area where they are installed by reducing the albedo to less than 1%. So they may save on CO2 emissions but they come with their own drawbacks, particularly if you live near them. And if they are added to roofs of buildings in cities and urban areas, they will substantially warm those areas.
Net effect on global warming: small increase in local temperatures.
iv) Heat production
There is one UHI effect that does significantly affect temperatures though: waste heat. This is where human energy use ends up as waste heat that heats the local environment around where the energy is being used. As I showed first in Post 14 and later in Post 29, this direct anthropogenic surface heating (DASH) can warm suburbs, cities and even whole countries by up to 1°C. But in fact even that warming is small compared to large cities like London.
In 2013 the total energy use in Greater London from all sources was estimated at over 150,000 GWh. That is equivalent to an average power consumption of over 15 GW throughout the year. As the area of Greater London is about 1569 km2, this amounts to a constant power density of 9.6 W/m2. In Post 14 I explained how increasing the 161 W/m2 of solar radiation absorbed by the Earth's surface by 2.25 W/m2 would be sufficient to increase the mean surface temperature by 1°C. But I also explained that any other source of heat that was absorbed or produced at the surface would have the same effect. So 2.25 W/m2 of waste heat generated at the surface would also lead to 1°C of warming.
In London the waste heat will amount to 9.6 W/m2, more or less the same as the total power usage. This is because, according to the second law of thermodynamics, all energy is destined to end up as heat or entropy eventually. So waste heat is probably responsible for over 4°C of warming in London - not a great shock to people who live there. That is the urban heat island effect (UHI).
Of course not everyone sees it this way. In climate science this warming is dismissed as trivial because it only amounts to 0.028 W/m2 of power use when averaged across the entire surface of the Earth, and so it only raises global mean temperatures by about 0.01°C. While this is technically correct, it neglects the uneven distribution of both these heat sources and the weather stations that determine the global temperature. Most weather stations are on land, almost 90% are in the Northern Hemisphere, and most of these are in the USA, Europe and China. So a high proportion are going to be distorted by the UHI effect from waste heat. That is what makes it important.
Net effect on global warming: large increase in large cities and much of Europe and the USA.
Summary
What I have shown here is that most types of urban heat island (UHI) have little or no effect on global warming with one exception: waste heat. This can add several degrees to the local temperatures.
However, even this is not the full story because the existence UHIs of themselves is not the only issue. Just because a small area of the Earth's surface retains or produces more heat than another does not mean that overall temperatures will rise and add to global warming. It is the change in heat retention and emission over time that is important, not the magnitude or difference from the rest of the environment. A UHI has no impact on global warming if its energy usage is not changing over time. Unfortunately in most cases the energy usage has changed, and by a large amount.
In the next six posts I will highlight six extreme examples of UHIs in the Southern Hemisphere. These are all examples of UHIs in large cities where the UHI temperature has increased much faster than that seen in the country or region as a whole, probably due to significant growth in the size, population and energy use in those cities.